ASPIRANTSHUB.IN
JEE-MAIN, JEE-ADVANCE,
NEET, MHT-CET every thing is here.
We provide you all the career options available and their scope in India in scienceStream.
As we are a team of Science Experts so we also guide you in preparation of Entrance-exams.
First Law of Thermodynamics And Its Application
1. Thermodynamic System:
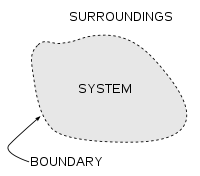
A thermodynamic system may be defined as definite amount of matter (solid liquid or gas) surrounded by some closed surface. Anything outside this system which can exchange energy with it is called its surrounding. Generally the state of every thermodynamic system is defined by three thermodynamic parameters, viz, pressure, temperature and volume.
1.
Thermodynamic
System:
A thermodynamic system may be defined as definite amount of matter (solid liquid or gas) surrounded by some closed surface. Anything outside this system which can exchange energy with it is called its surrounding. Generally the state of every thermodynamic system is defined by three thermodynamic parameters, viz, pressure, temperature and volume.
2.Thermodynamic Equilibrium:
The state of a system in which thermodynamic coordinates have definite values and they do not change as long as external conditions are unchanged is called the thermodynamic equilibrium state.
A system in thermodynamic equilibrium satisfies:
1. Mechanical equilibrium (no unbalanced forces)
2. Thermal equilibrium (no temperature differences)
3. Chemical equilibrium.
I am a text block. Click on me to drag me around or click a corner handle to resize me. Click the settings icon (it's the left one, looks like a cog) to change this text. You can type new text into me or cut and paste text from somewhere else. Click outside of me when you're done and any changes will be saved.

4.Equation of State:
An equation of state is a relation between the thermodynamics coordinates of a system at equilibrium. The general equation of state is given as f(p,v,t).
.
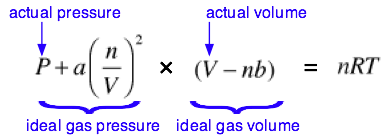
For an ideal gas the equation
state takes the form PV=NRT
![]() . For real gas there are various forms of
equation of state, the most well-known being Van der Waal’s
equation,
. For real gas there are various forms of
equation of state, the most well-known being Van der Waal’s
equation,
Where a and b are constants depending on the nature of gas.
5.Work done in various thermodynamic interaction:
( (a) Isothermal change: In this thermodynamic interaction temperature of the system remains constant. Hence dT= 0 for isothermal change.
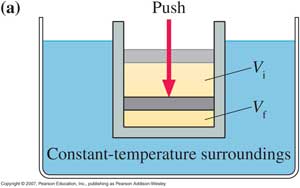
(b) Adiabatic change: In this case no heat is allowed to enter or leave the system. Therefore, dQ = 0 for adiabatic change

|
|
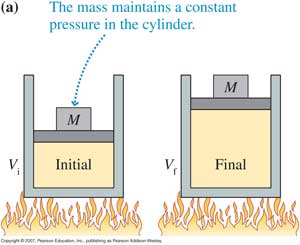
(c) Isobaric change : The pressure of the system remains constant during the interaction. Hence dP = 0 for isobaric change.
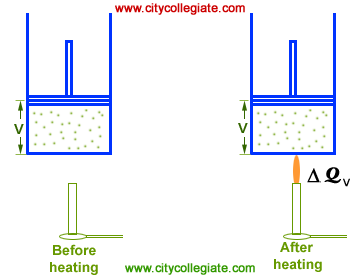
(d) Isochoric change : Volume of the system kept constant in isochoric change. Therefore, dV = 0. Hence work done by the system or on the system is zero in this case.










can u pls tell me the proper time management for a student who is preparing for both boards as well as entrance?
Experts;-
@aarushi
when I was in 12th standard, my main aim was to prepare for entrance exams. All the concepts you learned for entrance can easily be applied in board exams. In physics you just need to learn derivations which are not in entrance. So preparing for entrance exams should be your main priority and then preparing for board exams will be as easy as moving knife in the cake.